Probing the molecular mechanisms of GPCR functional selectivity in live cells
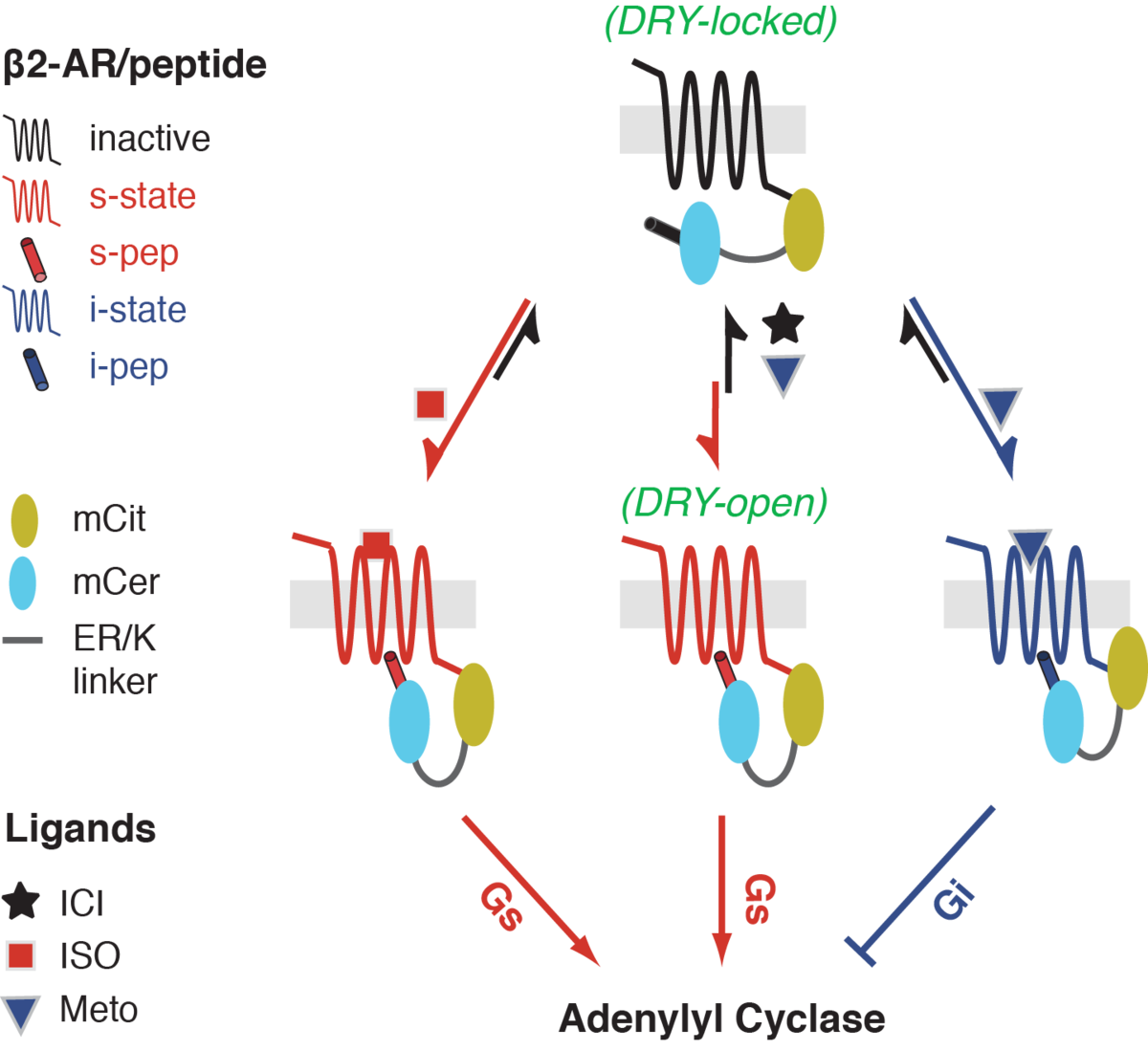
A fundamental unanswered question in GPCR signaling is the role of structural conformations of the GPCR in G protein selection. An emerging view from several studies is that GPCRs are not simple ‘on-off’ switches, but adopt multiple conformations in a ligand-dependent manner. In turn, these ligands elicit diverse functional responses through the activation of distinct G protein heterotrimers or G protein independent effectors such as arrestins. The conformational plasticity of GPCRs addresses the phenomenon of functional selectivity, wherein the same GPCR can elicit diverse ligand-dependent responses. Currently there is no direct method to link ligand-specific GPCR conformations observed in structural studies to differential downstream responses. The laboratory has used protein engineering to develop a GPCR conformational biosensor (Malik et al., JBC 2013) that can detect G protein-specific conformations. We have used this technology to demonstrate a Gi-specific conformation in the β2-adrenergic receptor induced by the β-blocker metoprolol (Fig. 1). Currently several laboratories are using our sensors to profile the functional selectivity of a range of GPCRs.
We have also used protein engineering to map the rules of the GPCR-G protein interaction. This study has yielded surprising insights into the affinities of GPCRs for G proteins, and the functional consequences of these interaction profiles in downstream responses. Our findings provide a strong foundation for GPCR-G protein pairing, and in due process have yielded a simple yet powerful genetically encoded technique to ‘tune’ G protein selection in live cells. Current efforts are focused on using this technique to (a) link G protein selection to physiological responses in cells and model organisms; and (b) define a quantitative ‘bias factor’ for ligands that is independent of the cell type being examined.
Selected Publications
- β2-adrenoceptor ligand efficacy is tuned by a two-stage interaction with the Gαs C-terminus. Kim K, Paulekas S, Sadler F, Gupte TM, Ritt M, Dysthe M, Vaidehi N, Sivaramakrishnan S. Proc Natl Acad Sci U S A. 2021 in press
- Minute-scale persistence of a GPCR conformation state triggered by non-cognate G protein interactions primes signaling. Gupte TM, Ritt M, Dysthe M, Malik RU, Sivaramakrishnan S. Nature Comm. 2019 Oct 23;10, 4836
- Priming GPCR signaling through the synergistic effect of two G proteins. Gupte TM, Malik RU, Sommese RF, Ritt M, Sivaramakrishnan S. Proc Natl Acad Sci U S A. 2017 Apr 4;114(14):3756-3761
- Structural elements in the Gαs and Gαq C-termini that mediate selective GPCR signaling. Semack A, Sandhu M, Malik RU, Vaidehi N, Sivaramakrishnan S. J Biol Chem. 2016 Aug 19;291(34):17929-40
- Detection of G Protein-selective G Protein-coupled Receptor (GPCR) Conformations in Live Cells. Malik RU, Ritt M, Devree BT, Neubig RR, Sunahara RK, Sivaramakrishnan S. J Biol Chem 2013 Jun 14;288(24):17167-78