Emergence of collective behavior in the myosin family of molecular motors
Myosin function in cells emerges from the coordinated interaction of multiple myosins and actin filaments. Despite the importance of myosins in muscle contraction and cellular architecture, the principles governing collective actin-myosin behavior are just beginning to be understood. My lab integrates both theoretical/numerical modeling and experimental measurements in motor ensembles, patterned precisely with DNA nanotechnology, to define the physical parameters that govern collective behavior.
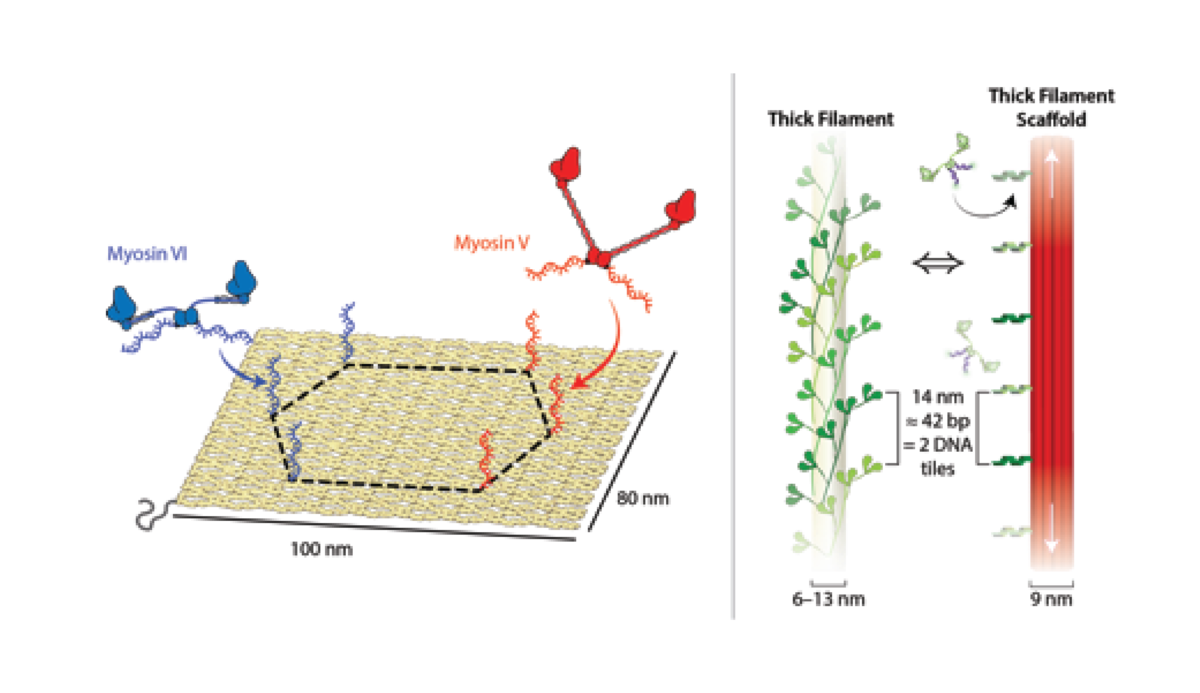
Our studies with myosin V and VI identified a novel role for the myosin lever arm in influencing collective movement trajectories (Fig. 1, Hariadi et al, PNAS, 2014). Myosin V and VI co-localize to membrane vesicles in neuronal growth cones. Our studies reveal an elegant mechanism for sorting of membrane vesicles, simply by controlling the relative number of motors in a vesicle population (Hariadi et al, eLife, 2015). Ongoing research in the lab is focused on the reconstitution of the myosin-cargo protein complex to understand the cellular regulation of unconventional myosins.
We have also developed a synthetic myosin filament to study mechanical coordination in muscle myosins. By pairing experimental measurements and stochastic simulations, we provide definite evidence for mechanical communication between multiple myosin heads in a large myosin ensemble (Fig. 1; Hariadi et al; Nature Nanotechnology, 2015). Current efforts are focused on reconstituting the intact thick-thin filament interface akin to muscle. In addition to dissecting the role of individual proteins on the collective acto-myosin interaction, we are also interested in investigating the effects of mutations in thick/thin filament proteins implicated in hypertrophic cardiomyopathy (HCM). HCM affects up to 1 in 500 individuals worldwide and currently there are no specific therapies for this disease condition, in part due to our limited understanding of the effects of disease mutations on collective acto-myosin contractility.
Selected Publications
- Dynamic multimerization of Dab2-Myosin VI complexes regulates cargo processivity while minimizing cortical actin reorganization. Rai M*, Vang D*, Ritt M, Sivaramakrishnan S. J Biol Chem. 2020;Dec 28;jbc.RA120.012703. (Editor's Pick)
- Stiffness of Cargo–Motor Linkage Tunes Myosin VI Motility and Response to Load. Shrivastava R, Rai M, Salapaka M, Sivaramakrishnan S. Biochemistry. 2019 Sept 11;58, 47, 4721–4725 (Featured Cover Article)
- Engaging myosin VI tunes motility, morphology, and identity in endocytosis. Ritt M, Sivaramakrishnan S. Traffic. 2018 Jun 4; 19:710–722
- Mechanical coordination in motor ensembles revealed using engineered artificial myosin filaments. Hariadi RF, Sommese RF, Adhikari AS, Taylor RE, Sutton S, Spudich JA, Sivaramakrishnan S. Nat Nanotechnol. 2015 Aug;10(8):696-700
- Myosin lever arm directs collective motion on cellular actin network. Hariadi RF, Cale M, Sivaramakrishnan S. Proc Natl Acad Sci U S A 2014, 111(11), 4091-6.